Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Food-Water-Energy Nexus for (big) children and Scepticals
*Corresponding author:Caroline Samberger, Stantec Institute for Water Technology and Policy Stantec - Buckingham Court, Kingsmead Business Park, Frederick Place, London Rd, High Wycombe HP11 1JU, UK.
Received: April 30, 2022; Published: June 16, 2022
DOI: 10.34297/AJBSR.2022.16.002250
The Beginning of Life
The Origins
Our body is made of approximately 7 000 000 000 000 000 000 000 000 000 atoms on average. Living organisms on Earth are primarily made of six elements: O (Oxygen), C (Carbon), H (Hydrogen), N (Nitrogen), P (Phosphorus) and S (Sulphur). Most of these elements were born in dying stars billion years ago.
Law of conservation of mass
In the 18th century, Antoine Lavoisier established that “matter on our planet is conserved”: “Matter can be changed from one form into another, mixtures can be separated or made, and pure substances can be decomposed but the total amount of mass remains constant.”
Between biotic and abiotic worlds
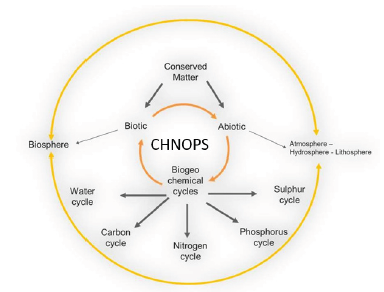
Elements like C, H, N, O, P, and S are the building blocks of life on Earth. The ways in which an element – or in some cases, a compound of elements such as water – moves between its various living and non-living forms and locations is called a biogeochemical cycle.
Based on the law of conservation of mass, elements move between the biotic (living world/biosphere) and abiotic world (non-living composed of the atmosphere, hydrosphere and lithosphere) (Figure 1).
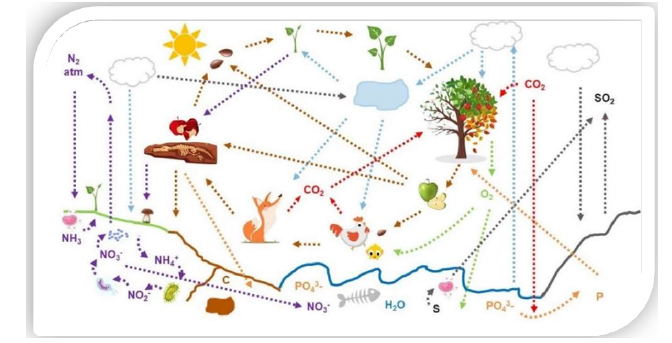
Figure 2 summarises in a nutshell the complexity of the interactions between the biotic and the abiotic worlds and between the different cycles. However, these primary six elements are finite resources on Earth…: what is taken from one cycle may end up in another cycle and under another physical form.
Carbon: The Building block of Life
Our body
Our body is made up of 18 % weight carbon which for an average sized human represents around 8x1026 carbon atoms at any one given time. Most of the carbon and nitrogen in our bodies comes from the food we eat [1].
The carbon we exhale in CO2 is the carbon that decomposes in our bodies. Eating brings new atoms to our body to replace the ones we are constantly losing. Earth is a closed system which means we literally all share the same planet, the same biosphere and more importantly the same atoms.
Our food
Nutrients in food are the materials the body needs to build itself
and stay in top working condition. Some of these nutrients provide
energy [2] and contain lots of carbon’ atoms:
a. Carbohydrates are a family of compounds made solely of
the three elements carbon, hydrogen, and oxygen. Glucose,
sucrose, fructose, maltose, and lactose are common sugars.
b. Lipids (fats) are a large family of compounds that are made
mostly of the elements carbon and hydrogen, with a small amount of oxygen.
c. Proteins (amino-acids) have the general formula RCH(NH2)
COOH. If carbohydrates and fat are the body’s energy sources,
proteins are the body’s building blocks.
Production, transportation and distribution of food also require energy and water. In 2014, the water footprint of agriculture represented 68 % of the global water footprint [3].
Our energy
In 2020, fossil fuels - carbon atoms stored in the Earth underground - still supplied 84 % of the World Energy. Production of energy also requires water and sometimes crops (for biofuels production), competing with harvesting, food production and land use. In 2014, the water footprint of energy production represented 12 % of the global water footprint [3]. About 4 % of the world’s agricultural land and 4 % of its fresh water are now used for growing biofuels [4]. By 2050, Earth population is predicted to reach 10 billion with an increase in energy demand of +80 %, food demand of +50 % and water demand of +50 % (Figure 2).
When it all goes wrong
Carbon cycle and carbon budget
Human activity, especially the burning of fossil fuels, has dramatically increased the exchange of carbon from the ground back into the atmosphere and oceans. Because Earth is a closed system, the amount of carbon on the planet never changes. However, the amount of carbon in a specific reservoir can change over time as carbon moves from one reservoir to another. As plants are eaten by herbivores and herbivores are eaten by carnivores, carbon moves up the food chain. Meanwhile, the respiration of plants, animals, and microbes returns carbon to the atmosphere as carbon dioxide (CO2). When organisms die and decay, carbon also returns to the atmosphere or is integrated into soil along with some of their waste.
While some phases of the carbon cycles can take only up to a year (i.e plant growth and decay), other carbon cycle pathways can take millions of years (i.e fossil fuel formation), so much so that not all carbon exchange cycles have the same duration and not all carbon forms are recycled back to where they came from in the biogeochemical cycles. Unfortunately, in the period post industrial revolution, carbon (essentially under the form of fossil fuel) was extracted from the slow carbon cycle at a faster pace than it could be reabsorbed. In parallel, deforestation due to agriculture accelerated tremendously, destroying potential natural carbon sinks and contributing to climate change.
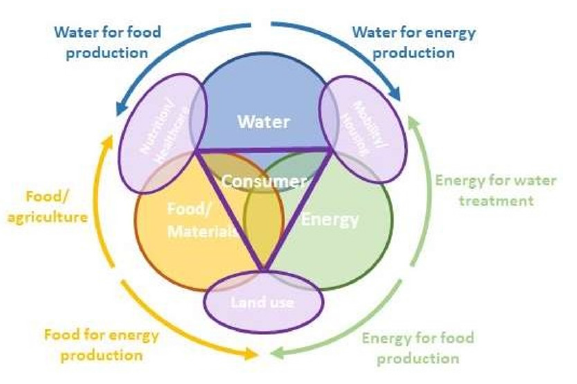
The food-water-energy nexus
Humanity’s ability to fulfil its basic needs is based on the availability of 3 main commodities - food/materials, energy and water - which are strongly interlinked: water is necessary to produce, transport and use all forms of energy; and energy is required for the extraction, treatment and distribution of water, as well as its collection and treatment after use. In the same way, both water and energy are also required to produce food. And food/ crops used to produce energy in the form of biofuels [3] (Figure 3).
That implies that the choices made in one domain have direct
and indirect consequences on the others:
By 2050, Humanity will need increased resources. The decision
to give priority to one commodity rather than the 2 others lie at
the heart of what has become known as the “water-food-energy”
nexus. The nexus affects the extent to which water, energy and food
security objectives can be simultaneously achieved by Humanity to
ensure sustainability for the future generations [4].
References
- Forbes (2020) How many atoms do we have in common with one another?
- National Library of Medicine (1992) Eat for life: The Food and Nutrition Board’s guide to reducing your risk of chronic disease. Chapter 3, The Food We Eat.
- Samberger C (2022) The role of water circularity in the food-water-energy nexus and climate change mitigations. 6:100061
- (2016) Fuel or food? Study sees increasing competition for land, water resources, ScienceDaily.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.